Patient & Advocacy Resources

Supporting Patients and Families
We welcome new partnerships with:
Cibus Therapeutics is committed to improving the lives of individuals affected by chronic pancreatitis (CP) and inflammatory bowel disease (IBD), including ulcerative colitis. We understand that these conditions can be physically and emotionally challenging—not only for patients but also for their families and caregivers.
That’s why we strive to be more than a biotech company—we’re a trusted partner in the patient journey. Through innovative research, accessible resources, and strategic collaborations, we aim to provide better treatment options, clearer information, and renewed hope. At every step, we’re here to support patients in managing their condition and improving their quality of life.
Resources for Patients
Chronic Pancreatitis?
Chronic Pancreatitis (CP) is a long-standing inflammation of the pancreas that leads to irreversible damage of the pancreatic tissue. Unlike acute pancreatitis, which is sudden and may resolve, chronic pancreatitis is progressive and can severely impact both digestive and endocrine functions.
Symptoms
- Persistent abdominal pain (often radiating to the back)
- Nausea and vomiting
- Unintended weight loss
- Steatorrhea (fatty, foul-smelling stools)
- Diabetes (due to loss of insulin-producing cells)
Causes
- Long-term alcohol use (most common)
- Genetic mutations (e.g., PRSS1, SPINK1, CFTR)
- Autoimmune conditions
- Obstructive causes (e.g., pancreatic duct strictures or stones)
- Idiopathic (unknown cause)
Current Treatment Options
There is currently no cure for chronic pancreatitis. Treatment focuses on symptom management and preserving pancreatic function:
- Pain management (analgesics, nerve blocks)
- Pancreatic enzyme replacement therapy (PERT) for digestion
- Nutritional support (low-fat diet, vitamin supplementation)
- Endoscopic or surgical interventions to remove obstructions or necrotic tissue
- Diabetes management, if endocrine function is impaired
Emerging therapies like CBT-101 aim to target the underlying inflammatory pathways to halt disease progression and offer long-term relief.
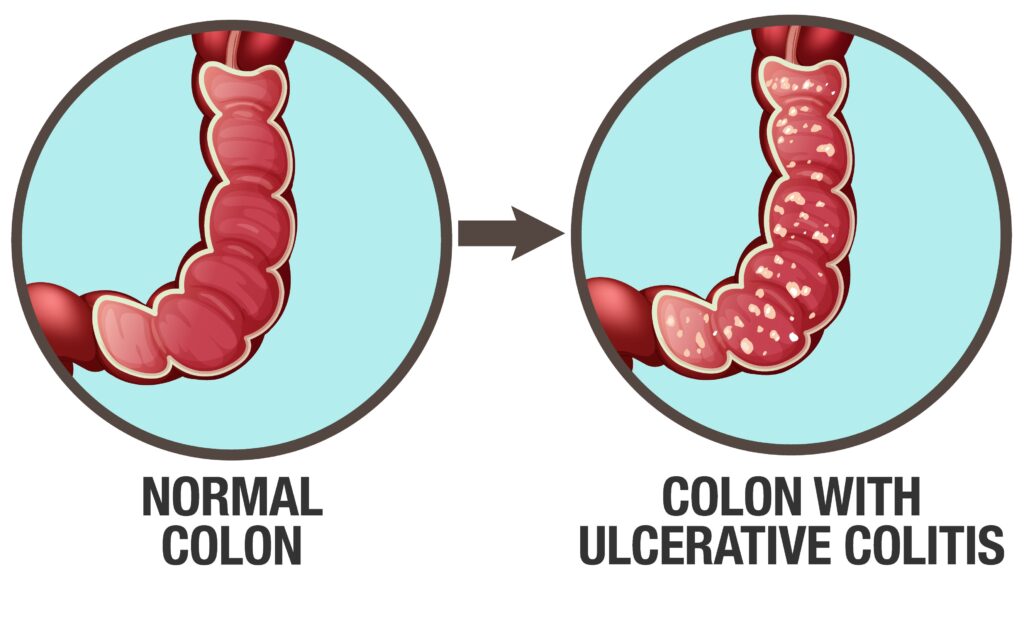
Ulcerative Colitis
Ulcerative Colitis (UC) is a chronic, relapsing-remitting inflammatory bowel disease (IBD) characterized by inflammation and ulceration of the inner lining of the colon and rectum. It typically presents in early adulthood but can occur at any age.
Symptoms
- Abdominal pain and cramping
- Bloody diarrhea
- Urgency to defecate
- Fatigue
- Weight loss
Causes
The exact cause of UC remains unknown, but it is believed to involve:
- Immune system dysregulation
- Genetic susceptibility
- Environmental triggers (e.g., diet, antibiotics)
Current Treatment Options
Treatment is aimed at inducing and maintaining remission:
- Aminosalicylates (5-ASAs) like mesalamine
- Corticosteroids for short-term inflammation control
- Immunomodulators (azathioprine, 6-MP)
- Biologics (anti-TNF agents, anti-integrins)
- JAK inhibitors
- Surgery (colectomy) in refractory cases
Oral small molecules like CBT-101 are in development to offer safer, more effective, and convenient alternatives that broadly modulate inflammatory pathways like JAK/STAT and NLRP3.
Clinical Trial Participation
Participating in a clinical trial is a meaningful way to contribute to medical research while gaining access to promising new therapies. At Cibus Therapeutics, we are preparing for First-in-Human trials of CBT-101, and your participation may help shape the future of GI disease treatment.
What to Expect
- Screening Process: Eligibility is determined through medical history, lab tests, and physical exams.
- Informed Consent: Participants receive detailed information about the trial, including potential risks and benefits.
- Study Visits: Regular check-ins, tests, and monitoring will be required.
- Compensation: May include travel reimbursement and compensation for time and participation.
Benefits of Participation
- Access to cutting-edge therapies not yet available to the public
- Close medical monitoring by leading specialists
- Contributing to the development of future treatments
Participation is voluntary, and you can withdraw at any time.


